
Author: Chris Taylor
Author Bio
Chris Taylor, Senior Engineer of Aeson Power, specializing in sodium-ion cell design and performance optimization. His research focuses on high-rate discharge behavior, thermal stability, and real-world operating conditions for sodium-ion batteries.
Introduction
As a battery engineer working on sodium-ion cell design and performance optimization at Aeson Power, I am often asked why the industry is paying increasing attention to sodium-ion technology. From my perspective, the renewed interest is not driven by a single performance metric, but by a growing mismatch between real-world application demands and what conventional batteries are optimized for.
Sodium-ion batteries are rechargeable energy storage systems that operate on principles similar to lithium-ion batteries, relying on reversible ion movement between two electrodes. The key difference is that sodium ions replace lithium ions as the charge carriers. While this may sound like a simple substitution, in practice it changes how the battery behaves in terms of power delivery, temperature response, and long-term stability.
How are Sodium-Ion Batteries Different?
In my work, one of the first things I emphasize is that sodium-ion batteries are designed around fundamentally different priorities than conventional battery technologies, including both lithium-ion and lead-acid batteries.
For example, lithium-ion batteries benefit from lithium’s small ionic radius and high electrochemical potential, which enable high energy density and compact system design. This makes them well suited for applications where weight and volume are the primary concerns. However, these advantages often come with trade-offs in cost sensitivity, temperature tolerance, and high-rate durability.
Lead-acid batteries, on the other hand, have long been valued for their low upfront cost, mature manufacturing processes, and robustness in simple charging systems. From an engineering perspective, however, their electrochemical reactions are highly sensitive to temperature and operating conditions. At low temperatures or under high current demand, lead-acid batteries experience rapid increases in internal resistance, reduced available power, and accelerated degradation mechanisms such as sulfation and electrolyte stratification.
Sodium-ion batteries occupy a different position altogether. Rather than optimizing purely for energy density or minimum cost, they are designed to prioritize stable power delivery, wide temperature adaptability, and long-term performance consistency. Sodium ions, being larger, interact differently with electrode materials and impose different design constraints, but these same characteristics also enable more stable ion transport and internal resistance behavior under demanding conditions.
As a result, higher energy density does not automatically translate into better real-world performance. In many applications I work with—particularly those involving cold environments, high cranking currents, or frequent cycling—what matters most is whether the battery can consistently deliver power, tolerate temperature extremes, and maintain stable performance over time. Sodium-ion batteries are inherently well suited to these requirements.
Inside the Structure of a Sodium-Ion Battery
From a cell design standpoint, sodium-ion batteries rely on material systems optimized for structural stability and ion transport rather than maximum energy density. This represents a clear departure from both lithium-ion systems, which prioritize compact energy storage, and lead-acid systems, which rely on bulk electrode mass and liquid electrolyte chemistry.
On the cathode side, materials such as Prussian Blue analogs and sodium-based phosphates are commonly used. I value these materials because their open crystal frameworks allow sodium ions to move in and out with less structural stress during cycling, contributing to stable performance and long service life.
On the anode side, hard carbon is the practical choice. In my experience, graphite—widely used in lithium-ion batteries—does not accommodate sodium ions efficiently. Compared to the lead-based negative electrodes used in lead-acid batteries, hard carbon offers far greater flexibility in ion storage mechanisms. Its disordered structure and diverse storage sites enable more stable sodium-ion storage, especially under high-rate discharge or low-temperature conditions.
The electrolyte system is equally critical. While lead-acid batteries rely on aqueous electrolytes that are highly temperature-dependent, and lithium-ion systems often suffer from reduced ion mobility in cold environments, sodium-ion electrolyte formulations can be optimized to maintain more stable ion transport across a wider temperature range. I pay close attention to electrolyte design because interfacial stability and ion mobility largely determine how the cell behaves outside laboratory conditions, particularly in real-world cold-start and high-power applications.
How Sodium-Ion Batteries Work
To understand why sodium-ion batteries behave the way they do, it helps to look closely at their operating mechanism. At a fundamental level, a sodium-ion battery is a reversible electrochemical system controlled by ion transport, electrode structure, and interface stability.
During charging, an external power source drives sodium ions out of the cathode and toward the anode through the electrolyte, while electrons flow through the external circuit. During discharge, this process reverses, allowing the battery to deliver electrical energy. In my work, the speed and stability of sodium-ion movement are key factors that determine power capability and efficiency.
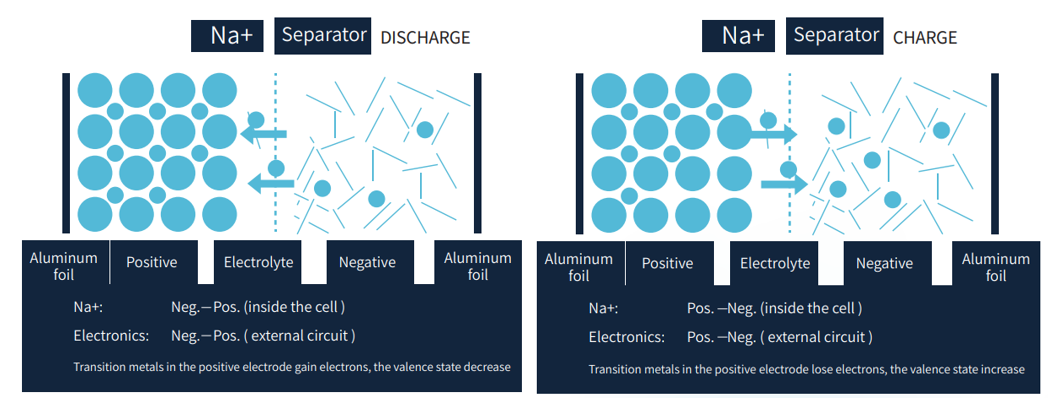
Working Principle of Sodium-ion Battery
Key Features and Automotive Applications
At Aeson Power, I work on designing and optimizing sodium-ion batteries. From my experience, the most noticeable advantages are instant power delivery, high safety margins, and reliable performance across a wide temperature range. These advantages position sodium-ion batteries to play a vital role in the automotive sector.
I will share how Aeson Power sodium battery is able to meet the real-world demands of modern vehicles.
Instant power delivery: “Whether it’s a cold winter morning or a high-load situation, the battery delivers strong cranking current immediately, ensuring reliable engine starts every time.”
High safety margins: “The batteries use stable polyanionic cathode materials with robust crystal structures, which resist structural collapse or thermal runaway even under high temperatures or abusive conditions. This makes them inherently safer than many conventional chemistries.”
Wide temperature operation: “With stable internal resistance and efficient ion transport from extreme cold to high heat, our batteries ensure reliable vehicle performance in any climate.”
Looking ahead, sodium-ion batteries are set to drive the future of automotive power, delivering reliable, safe, and all-weather performance for next-generation vehicles.















